
Mohannad J. Kadhum*, Jack E. Kochevar, Ben Koch, Ryan K. Swanson, Steve W. Laninga
*Corresponding author: mohannad.kadhum@lifecore.com
Sterile filtration using 0.2um membranes is a widely used method to eliminate the microbial load in a wide range of pharmaceutical formulations. However, sterile filtration of highly viscous solutions is challenging due to low throughput and potential concentration changes stemming from solution viscosity, retention, or adsorption. Due to the inherent difficulty in filtration of relatively large molecular weight polymers or highly concentrated biologics, other sterilization methods such as ebeam, gamma radiation, dry heat, autoclave, and others are usually implemented, if applicable. These methods may result in undesirable product attribute changes. Sterile filtration is usually restricted by manufacturer operating ranges of single-use cartridges and capsules. The limitation of using such filters are usually low operating pressure, up to 60 to 90 psi, in addition to significant holdup or concentration changes by size exclusion or adsorption.
In this work, high-pressure sterile filtration (HPSF) is presented as a method to increase yield without compromise on patient safety or product quality. A validated HPSF system that adheres to PDA and ASTM sterile filtration guidance was utilized (1, 2). This system has been used to filter sterilize sodium hyaluronate (NaHy) solutions used as ophthalmic viscoelastic devices (OVD), which are used in cataract surgery (nominal viscosities of 50,000 cps). Recently, the application of HPSF has expanded to other highly viscous solutions. The HPSF system runs up to a 900 psi for filtration of highly viscous formulations of NaHy, sodium carboxymethyl cellulose(Na-CMC), and other widely used excipients. Formulations of NaHy with molecular weights ranging from 900 up to 2,000 kDa with concentrations as high as 20g/L were examined. When comparing the filtration yield of a 10g/L NaHy solution with 900kDa molecular weight on a capsule filter at the maximum operating pressure (60 psi) set by the manufacturer to a HPSF with nearly equivalent filtration surface area of 200 cm , the flux on the high-pressure filter was about three orders of magnitude faster for the same solution. Furthermore, when comparing the NaHy concentration of filtrate to the feed using the HPSF to off-the-shelf filter cartridges, the HPSF maintained the concentration and viscosity of the feed solution, while a single cartridge caused 7% drop in concentration and up to 15% when redundant filtration was used. This clearly demonstrates the benefits of HPSF when filtering NaHy formulations.
Experiments conducted using solutions of 10g/L of NaHy of 900kDa molecular weight were filtered at pressures ranging from 45 up to 600 psi. Figure 1a shows that while filtration at pressures up to 95 psi resulted in low throughputs, high pressure continued to filter without plugging. Figure 1b shows that the maximum throughput at 600 psi was about two orders of magnitude higher than the throughput at 45 psi.
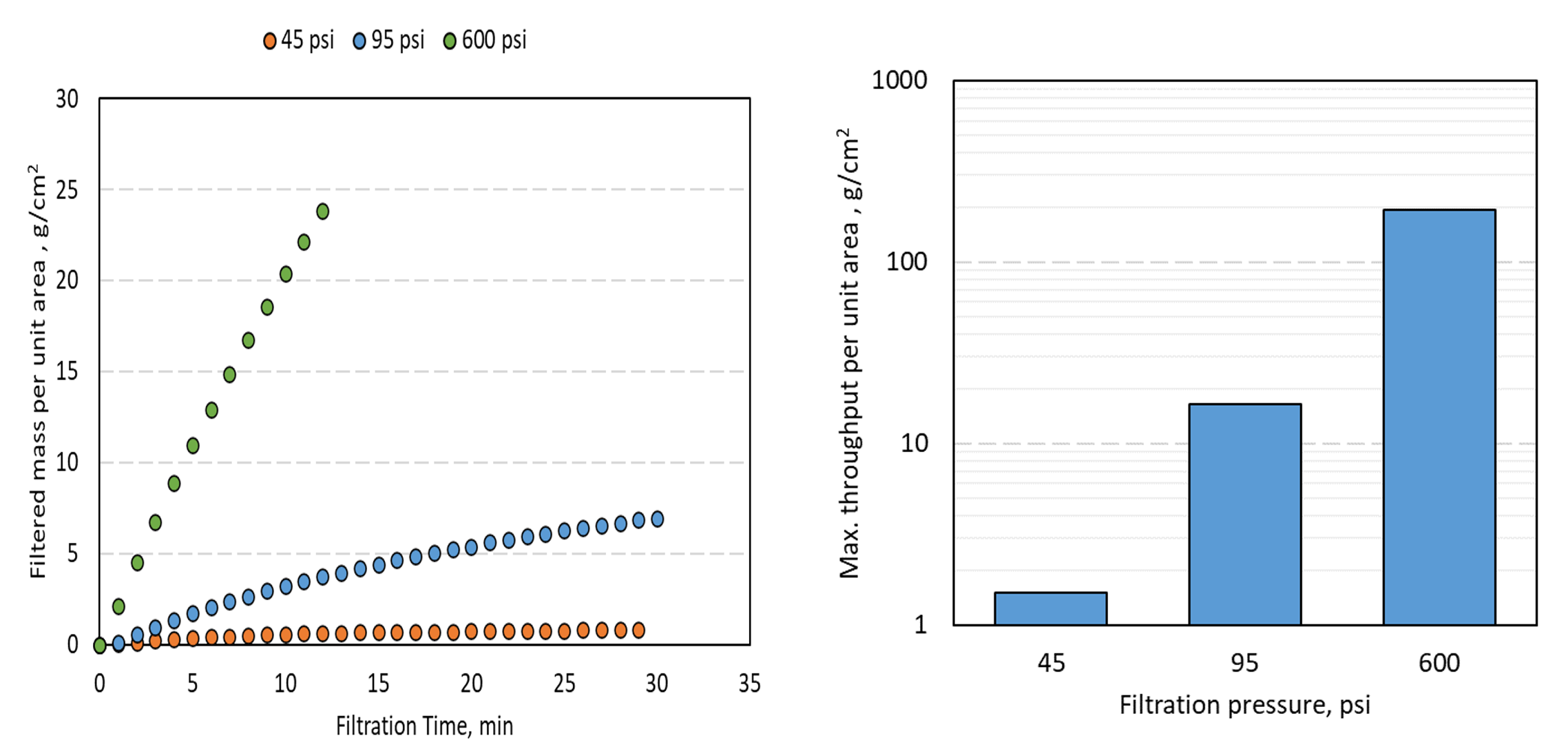
Figure 1
Sterile Filtration Throughput of NaHy solution of 900kDa and 10g/L concentration at varying filtration pressures a) filtered mass per unit filter area vs time of filtration b) comparison of the maximum throughput achieved at three filtration pressures.
A 20g/L NaHy solution with a viscosity of about 300,000 cp was successfully filtered at 800 psi, but was mainly unfilterable at low pressure of 45 and 95 psi as shown in Figure 2a. The viscosity of both unfiltered solution and filtrate of 800 psi filtration was the same as shown in Figure 2b, indicating no concentration change or retention
by the membrane.
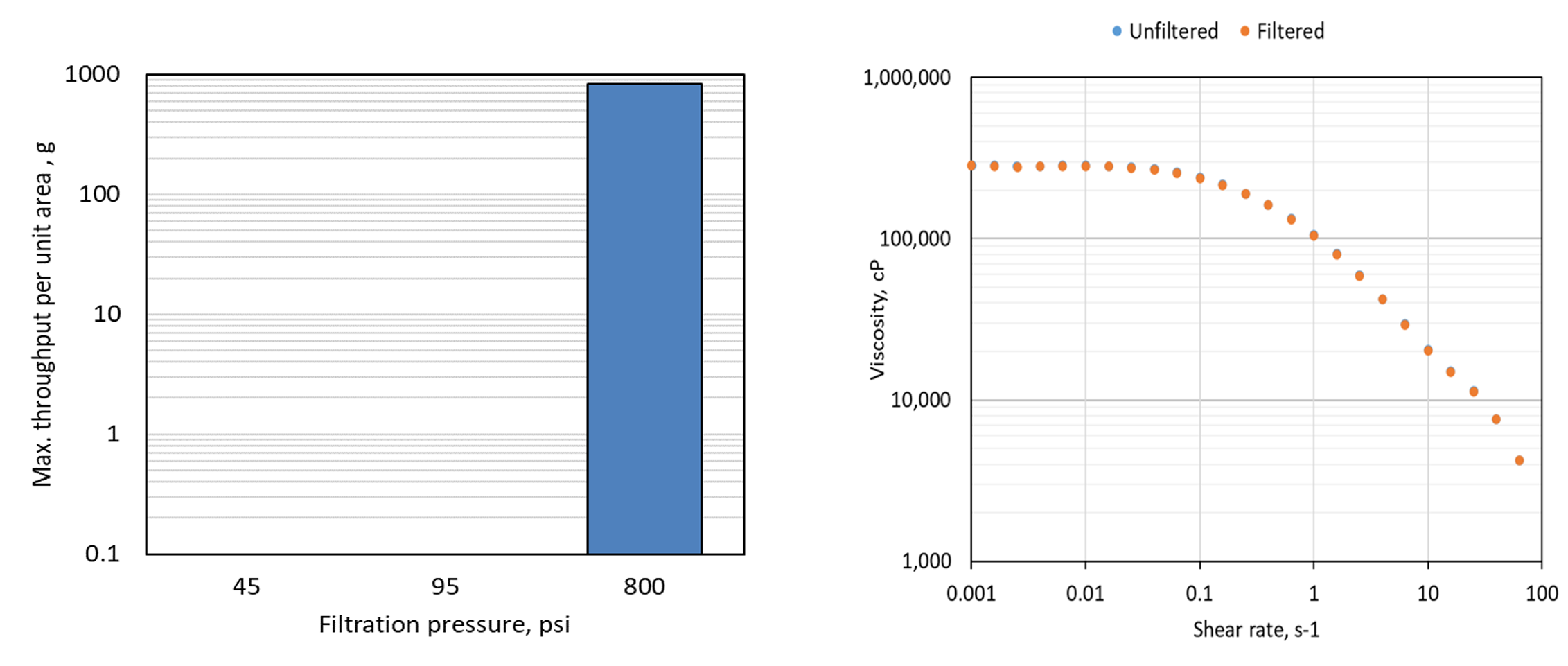
Figure 2
Sterile Filtration Throughput of NaHy solution of 900kDa and 20g/L concentration at varying filtration pressure: a) filtered mass at range at tested pressures; b) flow curves of unfiltered sample and 800 psi
filtrate.
A 20g/L NaHy solution with a viscosity of about 300,000 cp was successfully filtered at 800 psi, but was mainly unfilterable at low pressure of 45 and 95 psi as shown in Figure 2a. The viscosity of both unfiltered solution and filtrate of 800 psi filtration was the same as shown in Figure 2b indicating no concentration change or retention
by the membrane.
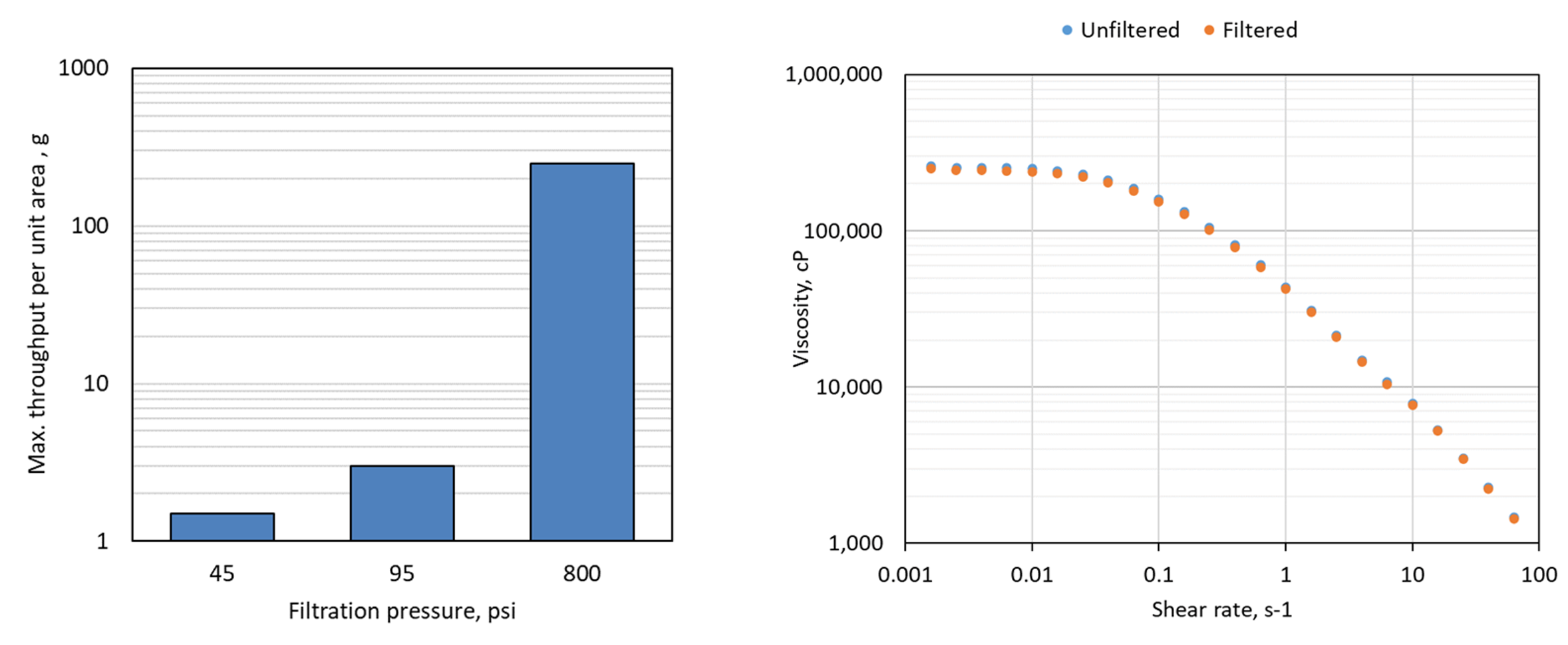
Figure 3
Sterile Filtration Throughput of NaHy solution of 2000kDa and 10g/L concentration at varying filtration pressure: a) maximum throughput achieved; b) flow curves of unfiltered sample and 800 psi filtrate.
Generally, the data above demonstrates that high pressure filtration is successful in filtering samples without a change in concentration. In comparison at low pressure, filtering a 10g/L solution of 900kDa molecular weight NaHy using an off the shelf cartridge with ≈2700 cm2 effective surface area resulted in significant retention of NaHy. Figure 4 below shows that a drop of about 25% in concentration was encountered when measured using a loss on drying(LOD) method using a halogen moisture analyzer. The concentration of samples collected during the entire filtration did not reach feed concentration suggesting constant retention of NaHy which can significantly complicate manufacturing processes.
Retention of polymer and concentration change complicate design of pharmaceuticals formulation at low pressure especially when redundant filtrations (two filters in series) are used.
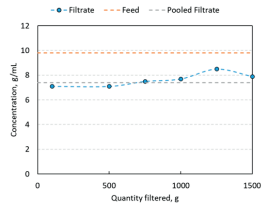
Figure 4
Concentration change between filtered and unfiltered solution at low pressure of 40 psi using 2700cm PES filter cartridge.
Formulations of Na-CMC with molecular weights of 250kDa and 700 kDa with 0.7-1.2 degree of substitution of carboxymethyl and up to 20g/L in concentration were also examined. A 2% CMC formulation has, on average, a one-order-of-magnitude higher throughput utilizing the HPSF system operated at 600 psi in comparison to filtration at 95 psi utilizing equivalent filtration surface areas as shown in Figure 5.
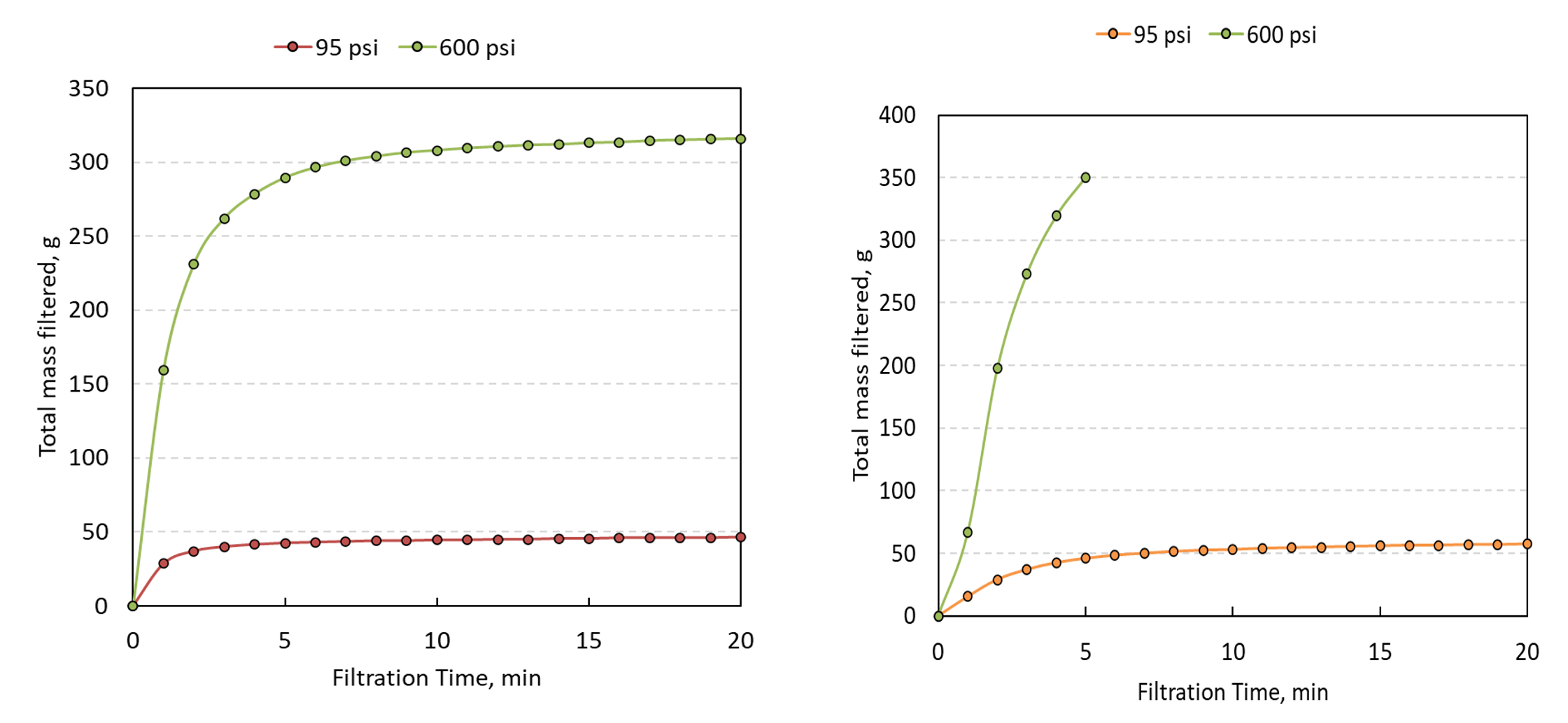
Figure 5
Filtration of NaCMC solution at range of pressures a) 5g/L NaCMC with 0.7 degree substitution b) 20g/L with 1.2 degree substitution.
In summary, sterile filtration of highly viscous pharmaceutical formulations using HPSF system can have clear advantages in terms of higher throughput, higher flux, and reproducibility in terms of concentration; and lower material holdup which can be beneficial when handling costly formulations. High pressure sterile filtration can fast-track development efforts and accelerate bringing early-phase formulations into the clinic.
References
Standard Test Method for Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration, ASTM International, F838-20(2020).
Sterilizing Filtration of Liquids, PDA Journal of Pharmaceutical Science and Technology, Technical Report No. 26 (2008).